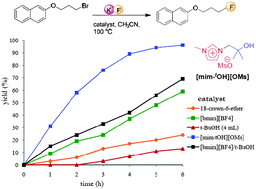
An ionic liquid bearing tert-butanol moiety ([mim-tOH][OMs]) was employed as an organocatalyst in the nucleophilic fluorination of a variety of substrates containing halogen/sulfonate as a leaving group. Low reactive metal fluorides, including KF, were used as a fluoride source in the reaction. Ionic liquid [mim-tOH][OMs] has shown excellent selectivity in nucleophilic fluorination of 2-(3-bromopropyloxy)naphthalene with KF or CsF affording 2-(3-fluoropropyloxy)-naphthalene in high yields. Among the various solvents screened, the activity of alkali metal fluoride was found to be better due to the improved solubility of both the metal fluoride and [mim-tOH][OMs] in the acetonitrile media.