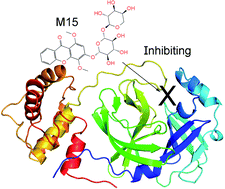
Preventing the biological activity of SARS-CoV-2 main protease using natural compounds is of great interest. In this context, using a combination of AutoDock Vina and fast pulling of ligand simulations, eleven marine fungi compounds were identified that probably play as highly potent inhibitors for preventing viral replication. In particular, four compounds including M15 (3-O-(6-O-α-L-arabinopyranosyl)-β-D-glucopyranosyl-1,4-dimethoxyxanthone), M8 (wailupemycins H), M11 (cottoquinazolines B), and M9 (wailupemycins I) adopted the predicted ligand-binding free energy of −9.87, −9.82, −9.62, and −9.35 kcal mol−1, respectively, whereas the other adopted predicted ligand-binding free energies in the range from −8.54 to −8.94 kcal mol−1. The results were obtained using a combination of Vina and FPL simulations. Notably, although, AutoDock4 adopted higher accurate results in comparison with Vina, Vina is proven to be a more suitable technique for rapidly screening ligand-binding affinity with a large database of compounds since it requires much smaller computing resources. Furthermore, FPL is better than Vina to classify inhibitors upon ROC-AUC analysis.