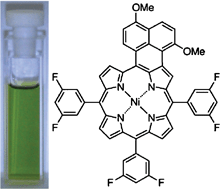
Oxidative aromatic coupling of meso-substituted porphyrins bearing one electron-rich naphthalene unit has been studied in detail. After thorough optimization of oxidant, naphthalene-fused porphyrins were prepared in high yield without contamination from chlorinated side-products using Fe(ClO4)3·2H2O. Copper and nickel complexes were successfully transformed into π-expanded porphyrins in 40–83% yield.