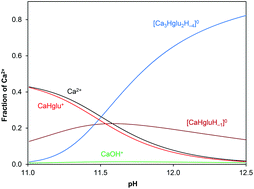
The equilibria and structure of complexes formed between the Ca2+ ion and the heptagluconate (Hglu−) ion in both neutral and alkaline solutions have been studied. In alkaline solutions an uncharged, multinuclear complex is formed with the composition of Ca3Hglu2(OH)4 (or [Ca3Hglu2H−4]0) with an unexpectedly high stability constant (lg β32–4 = 14.09). The formation of the trinuclear complex was deduced from