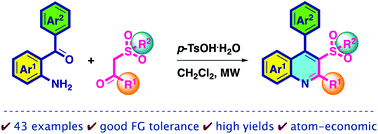
This study describes an efficient protocol for the preparation of substituted 2,4-diaryl-3-sulfonylquinolines from functionalized 2-aminobenzophenones and aromatic β-ketosulfones by using p-toluenesulfonic acid monohydrate under microwave irradiation. In this atom-economical synthetic route, a series of pharmaceutically active 3-arylsulfonylquinolines with good functional group tolerance are prepared in good to excellent yields. Some structures are confirmed by single-crystal X-ray diffraction analysis.