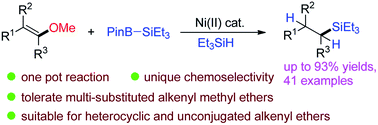
A new one pot protocol has been developed for the reductive silylation of alkenyl methyl ethers using Et3Si–BPin and HSiEt3 with nickel(II) catalyst. Styrene type methyl ethers, multi-substituted vinyl methyl ethers, heterocycles and unconjugated vinyl ethers are all tolerated to form alkyl silanes. Mechanistic study reveals that it is a cascade of a C–O bond silylation and vinyl double bond hydrogenation process. Internal nucleophilic substitution or oxidative addition pathways were both acceptable for C–O bond cleavage. The acquired intermediate alkenyl silanes then proceeded through an unconventional reduction process thus providing alkyl silanes.