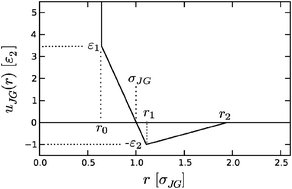
We examine the range of validity of the Gaussian model for various water-like liquids whose intermolecular potentials differ from SPC/E water, to provide insight into the temperature dependence of the hydrophobic effect for small hard sphere solutes. We find that low compressibility liquids that have more close-packed network structures show much larger deviations from Gaussian fluctuations for low or zero occupancies relative to more compressible fluids with more open networks. Water appears to be a unique molecular fluid in possessing equilibrium density fluctuations that are faithfully described by the Gaussian theory. We ascribe this success to the fact, shown here, that the orientational correlations near a small hard sphere solute involve remarkably little reorganization from the bulk, which is a consequence of water's low solvent reorganization enthalpy and entropy.