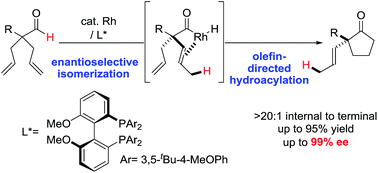
We describe a Rh-catalyzed desymmetrization of all-carbon quaternary centers from α,α-bis(allyl)aldehydes by a cascade featuring isomerization and hydroacylation. This desymmetrization competes with two other novel olefin functionalizations that are triggered by C–H bond activation, including carboacylation and bisacylation. A BIPHEP ligand promotes enantioselective formation of α-vinylcyclopentanones. Mechanistic studies support irreversible and enantioselective olefin-isomerization followed by olefin-hydroacylation.