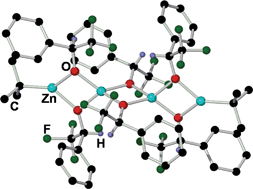
A systematic study of the stoichiometric alkylation reactions of 2,2,2-trifluoroacetophenone 1 with [ZnR2(TMEDA)] (R= Me, Et, tBu, CH2SiMe3; TMEDA= N,N,N′,N′-tetramethylethylenediamine) monitored by 1H and 19F NMR spectroscopy is presented. For R = Me, Et the alkylation products alkyl(alkoxides) [(TMEDA)Zn(R){OC(CF3)(R)Ph}] (R = Me, 2: Et, 3) are obtained as the single products of the reaction. When the steric bulk of the dialkylzinc reagent is increased the alkylation reaction is inhibited. Thus, for R = tBu, the reduction product [(TMEDA)Zn(tBu){OC(CF3)(H)Ph}] is obtained as a result of β-hydride elimination from one of the tBu groups of the organometallic reagent. 1H NMR spectroscopic monitoring of the reaction allowed the detection of isobutene as a side product of this reduction process. For the highly sterically demanding group R = CH2SiMe3 which lacks hydrogen atoms at the β position, no reaction is observed even under refluxing conditions. Two important intermediates from these reactions have been structurally elucidated: [(TMEDA)Zn(Me){OC(CF3)(Me)Ph}] (2) which could be involved in the previously reported alkylation reaction of trifluoromethyl ketones by ZnR2 catalysed by TMEDA and unprecedented tetranuclear [(tBu)2Zn4{OC(CF3)(H)Ph}6] (5) resulting from the reduction of 1 when reacted with tBu2Zn, which displays a rare Zn⋯Zn⋯Zn⋯Zn linear chain arrangement for a zinc alkyl(alkoxide).