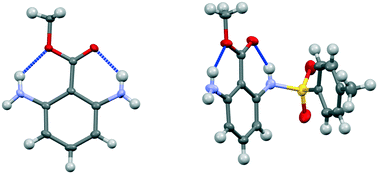
Methyl 2,6-diaminobenzoate and its bis-triphenylboron complex show hydrogen bonding from NH2 groups to both oxygen atoms of the carboxylic ester, and there is little difference in the lengths of these types of hydrogen bond, while the crystal structure of the tetrafluoroborate salt is dominated by cation/anion hydrogen bonding. In methyl 2-amino-6-tosylaminobenzoate, the more acidic tosyl-N–H group forms a hydrogen bond to the carbonyl and the primary amino group makes a hydrogen bond to the alkoxy group. A search of the Cambridge Structural Database reveals a number of examples of N–H⋯O hydrogen bonding to the alkoxy oxygen of an ester group with a preference for the N–H bond lying in the ester plane. Hydrogen bonding to the ester alkoxy group should not be excluded when considering mechanistic processes in chemical or biochemical systems.