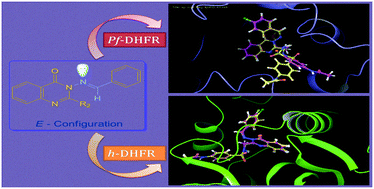
An optimization and modification of Grimmel's method leading to cyclization and incorporation of glycine linked sulphonamide at position-2 in 4-quinazolin-(3H)-ones was accomplished. Generation of a lipophilic site at position-3 of 4-quinazolinones was explored by synthesis of imines, unfortunately leading to an isomeric mixture of stereoisomers. These stereoisomeric mixtures were further converted to a single isomer utilizing the novel methodology developed by the use of an aprotic solvent system. Moreover, a mixture of (Z)/(E)-isomers and single configuration was identified and ascertained using NMR, HMQC, HMBC and NOESY spectroscopic techniques. The synthesized entities were further screened for their antimalarial efficacy pertaining two active scaffolds 8m and 8s. The active molecules were sent forth for enzyme inhibitory study against presumed receptors h-DHFR and Pf-DHFR computationally as well as in vitro, proving their potency as dihydrofolate reductase inhibitors. The oral bioavailability of these active molecules was also predicted by the study of ADME properties, indicating good bioavailability of the active entities.