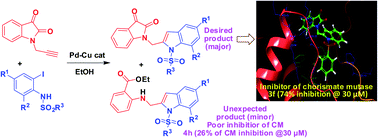
A series of novel isatin–indole derivatives has been designed as potential inhibitors of chorismate mutase (CM) that is known to be present in bacteria, fungi and higher plants but not in human. The design was supported by in silico docking studies that predicted strong interactions of these molecules with CM. The target compounds were synthesized via the one-pot coupling/cyclization method involving the reaction of an isatin based terminal alkyne with 2-iodosulfanilides under Pd–Cu catalysis. A number of isatin–indole derivatives were prepared using this method. A side product e.g. 2-indolylmethylamino benzoate ester derivative was obtained as a result of isatin ring opening (ethanolysis) of products in certain cases. Additionally, regioselective reduction of selected compounds afforded the corresponding C-3 hydroxy derivatives. All isatin–indole derivatives showed good to high inhibition of CM in vitro among which two compounds (3e and 3f) showed inhibition at nanomolar concentration.