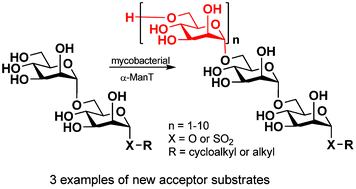
Three new (1 → 6)-α-D-mannodisaccharides with cyclohexylalkyl or octylsulfonyl function like aglycone were synthesized and screened in the mycobacterial mannosyltransferase assay. 2-Cyclohexylethyl (1 → 6)-α-D-Man2 acted as the best acceptor substrate, whereas the sulfonyl group significantly reduced the ability of the mannodisaccharide to serve as the acceptor. Despite these differences, mass spectrometric analysis confirmed the capability of all synthetic mannodisacharides to accept up to ten additional mannose units, i.e. the transfer was not affected by the type of aglycone. The results reported here suggest that the enzyme responsible for the consecutive mannose attachment is the processive α-mannopyranosyltransferase present in the cell-free system of the mycobacterial cell envelope.