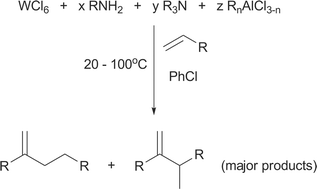
The selective dimerisation of the α-olefins 1-pentene through to 1-nonene is reported using an in situ-generated catalyst derived from tungsten hexachloride, aniline, triethylamine and alkylaluminium halide. The influence of reagent identity and reaction stoichiometry, along with activator, solvent and α-olefin substrate choice are probed. The catalyst is found to be highly selective towards dimerisation, minimising the formation of undesired heavier oligomers. Notably, the selectivity within the dimer fraction is found to favour the formation of products with methyl branches. The selectivity towards individual olefin isomers has been determined and the system is found to also produce trace levels of dienes and alkanes. A kinetic study of the system reveals a second order dependence on substrate. Comparison of the products observed, with those expected for metallacyclic and Cossee-type mechanisms, suggests that the latter is in operation, something confirmed by the results of a C2H4/C2D4 co-dimerisation experiment which showed full isotopic scrambling in the products. Thus a mechanistic proposal is made to account for the observed behaviour of the system, including the diene and alkane formation.