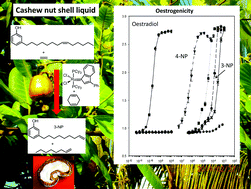
The ethenolysis of cardanol (2), a waste product from cashew kernel production, was carried out using a variety of metathesis catalysts. Surprisingly, the best activities and selectivities could be observed with ruthenium based 1st generation type catalysts converting cardanol (2) almost completely to the corresponding 1-octene (6) and 3-non-8-enylphenol (4), a potential detergent precursor. Detailed investigation of the reaction system showed that the high activity and selectivity were due to a combination of ethenolysis and internal self-metathesis of the unsaturated cardanol mixture, 2. Self-metathesis of cardanol (2) containing three double bonds led to the formation of 3-non-8-enylphenol (4) and 1,4-cyclohexadiene (7). The latter was crucial for a high selectivity and activity in the ethenolysis, not only of cardanol (2), but also of other substrates like methyl oleate (10) when using ruthenium based 1st generation catalysts. The endocrine disrupting properties of 3-nonylphenol and related compounds are compared.