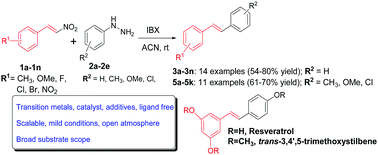
The generation of an aryl free radicals in the presence of nitrostyrenes through a combination of aryl hydrazines and o-iodoxybenzoic acid led to the synthesis of stilbenes by forming a new carbon–carbon bond after subsequent elimination of a nitrosyl radical. The symmetrical and unsymmetrical stilbenes with excellent E-selectivity were synthesized in good yields, with advantages of broad substrate scope and transition metal free, mild reaction conditions, under an open atmosphere in a short reaction time. A free radical mediated mechanism was postulated and supported by radical trapping experiments. Application of the developed methodology is demonstrated through a simple, two step synthesis of resveratrol, a valuable stilbenoid for anticancer and neurological studies via its prodrug E-1,3-dimethoxy-5-(4-methoxystyryl)benzene.