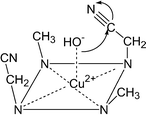
The metal promoted hydrolysis of nitrile groups in the side chains of tetraazamacrocyclic Cu2+ complexes has been studied by stopped-flow techniques. It is shown that the reaction proceeds by an intramolecular attack of an axially coordinated OH− onto the nitrile group to give the corresponding amide. In alkaline solution the amide then deprotonates and binds to the axial position of the Cu2+ thus preventing further coordination of an OH−. This explains mechanistically that in the Cu2+ complexes of macrocycles carrying two nitrile functions only one is selectively hydrolysed. The nitrile hydrolysis has also been used on a preparative scale to synthesize tetraazamacrocycles with two different side chains. X-Ray diffractions of several products are presented to confirm the structures and the results from the kinetics and equilibria measurements.