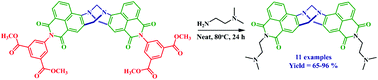
We report here a novel one-pot synthetic strategy for the synthesis of a family of N-alkyl-1,8-naphthalimide based Tröger's bases via a nucleophilic substitution reaction of a common ‘precursor’ (or a ‘synthon’) N-aryl-1,8-naphthalimide Tröger's base heated at 80 °C in neat aliphatic primary amine, in overall yield of 65–96%. This methodology provides an efficient and one-step facile route to design 1,8-naphthalimide derived Tröger's base structures in analytically pure form without the use of column chromatography purification, that can be used in medicinal chemistry and as supramolecular scaffolds. We also report the formation of the corresponding anhydride, and the crystallographic analysis of two of the resulting products, that of the N-phenyl-4-amino-1,8-naphthalimide and the anhydride derived Tröger's bases.