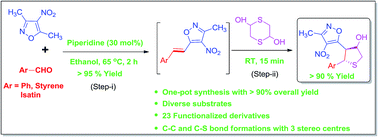
One-pot synthesis of highly functionalized tetrahydrothiophene (thiolane) derivatives conjugated with biologically useful isooxazole are reported via the Knoevenagel condensation followed by domino sulfa-1,6-Michael/intramolecular vinylogous Henry reactions of aldehydes, 1,4-dithiane-2,5-diol and 3,5-dimethyl-4-nitroisoxazole. From the base and solvent screening, it was found that piperidine (30 mol%) and ethanol are the most suitable conditions giving the desired products with >95% yields in 2–2.5 h overall reaction time.