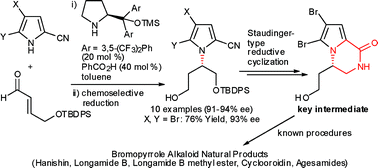
An unprecedented organocatalytic enantioselective formal synthesis of bromopyrrole alkaloid natural products is reported. An organocatalytic aza-Michael addition using pyrroles as the N-centered nucleophile is utilized as the enantioselective step to construct the nitrogen-substituted stereogenic carbon center in bromopyrrole alkaloids in good yield and excellent enantioselectivity. The aza-Michael product is converted via lactamization using a Staudinger-type reductive cyclization to the key intermediate, which was previously used in the total synthesis of bromopyrrole alkaloid natural products.