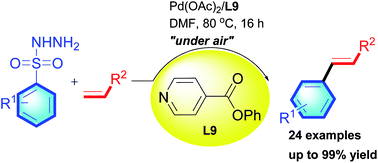
A palladium(II)-catalyzed oxidative Mizoroki–Heck reaction of arylsulfonyl hydrazides with alkenes was developed employing atmospheric air as the sole oxidant in an open-vessel manner. By using palladium(II) acetate associating with inexpensive, air-stable and moisture stable pyridine ligand L9 as the catalyst system, the efficiency of the reaction could be significantly enhanced. A wide range of arylsulfonyl hydrazides underwent the oxidative Mizoroki–Heck reaction with alkenes smoothly. Good-to-excellent product yields and excellent regio- and stereoselectivity were achieved. Functional groups such as halo, ester etc. were well-tolerated under these optimized reaction conditions.