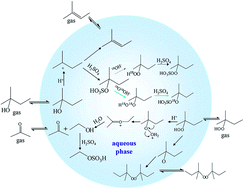
Organic hydroperoxides (ROOH) are reactive species which play significant roles in atmospheric processes, such as acid precipitation, hydroxyl radical cycling and secondary organic aerosol formation. Despite their observation in the atmosphere, our understanding of their formation mechanism is still incomplete. In the present work, ROOH formation was found in the acid-catalyzed heterogeneous oxidation of aliphatic alcohols with hydrogen peroxide. The kinetics and mechanism of acid-catalyzed heterogeneous oxidation of three aliphatic alcohols (2-methyl-2-butanol, 3-buten-2-ol and 2-butanol) with hydrogen peroxide were investigated. Based on the experimental results, tertiary or allyl alcohols may contribute to ROOH formation through this route while secondary alcohols may not. The kinetic experiments were conducted in a rotated wetted-wall reactor coupled to a mass spectrometer at room temperature (298 K) with 40–70 wt% H2SO4 solution. The reactive uptake coefficients were acquired for the first time. The generation and degradation mechanisms of ROOH in acidic media were proposed according to the product information. Once formed, ROOH are found to undergo two degradation pathways: the acid-catalyzed rearrangement reaction and the organic hydrogen peroxysulfate formation pathway. The newly found acid-catalyzed process may occur under certain conditions and influence particle growth in the atmosphere.