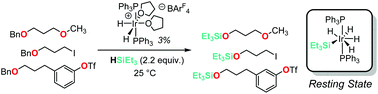
Catalysts capable of heterolytic silane activation have been successfully applied to the conversion of alkyl ethers to silyl ethers via C–O bond cleavage. The previously-reported cationic pincer-supported iridium complex for this transformation suffers from poor selectivity with regard to monodealkylation of substrate ethers. We demonstrate that a simple non-pincer iridium complex offers improved selectivity and is capable of benzylic ether cleavage in the presence of reductively-labile alkyl and aryl halide functionality. Preliminary mechanistic experiments suggest a neutral tetrahydridosilyliridium resting state which is consistent with previous mechanistic hypotheses. These experiments suggest that a pincer ligand framework is not required for activity in ether cleavage reactions and that simple cationic bis(phosphine)iridium complexes may offer improved selectivity profiles for applications to more-complex substrate molecules.