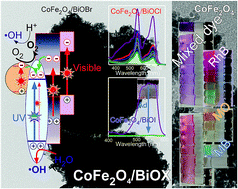
The recycling of photocatalysts and improving their activities by hybridizing two materials are important. Herein, nanosize ferromagnetic (Ms = 62.3 emu g−1) CoFe2O4 nanoparticles (NPs) were embedded into nanosize-assembled BiOX (X = Cl, Br and I) microflowers and examined by scanning electron microscopy, transmission electron microscopy, X-ray diffraction, UV-visible absorption spectroscopy, Fourier-transform infrared spectroscopy, and photoluminescence spectroscopy. The adsorption and photocatalytic performance of CoFe2O4/BiOX for methyl orange (MO), rhodamine B (RhB), methylene blue (MB), and a mixed dye (MO + RhB + MB) were examined under UV and visible light irradiation. The adsorption capacity of CoFe2O4/BiOI for RhB was 160 mg gcat−1, which is significantly larger than the <5 mg gcat−1 obtained for CoFe2O4/BiOCl and CoFe2O4/BiOBr. The photocatalytic activity was observed in the order of CoFe2O4/BiOBr < CoFe2O4/BiOCl < CoFe2O4/BiOI for RhB. Their adsorption and photocatalytic performances were also investigated with pure MO, pure MB and a mixed dye. MO in the mixed dye was the most easily removed by the catalysts under light exposure. Based on the scavenger tests, h+ and ˙O2− play major and minor roles in the photodegradation of the dyes, respectively. Although the ˙OH radical was formed for CoFe2O4/BiOBr and CoFe2O4/BiOCl, it has a much smaller role than the other active species.