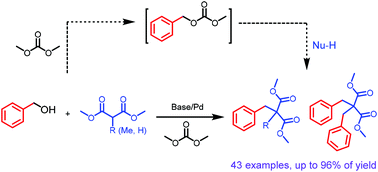
High step- and atom-economy are the endlessly pursued in organic and pharmaceutical syntheses. Herein, a new method for directly coupling benzyl/allyl alcohols with malonates via a palladium catalyzed Tsuji–Trost type reaction was developed. The reaction was carried out in an organic carbonate solvent which would activate alcohols in situ, replacing the traditional pre-synthesized carbonates. The new process demonstrated high efficiency, high selectivity and high generality. A wide variety of mono-substituted and bis-substituted malonates were selectively produced under different conditions, and this method represents a more step- and atom-economical and environmentally benign synthetic protocol.