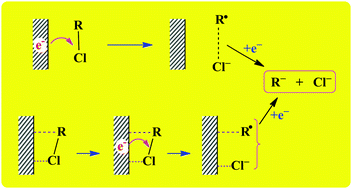
Dissociative electron transfer (DET) to a series of organic chlorides at glassy carbon (GC), silver and copper electrodes has been studied in 1-butyl-3-methylimidazolium tetrafluoroborate. The overall results of this study show that the ionic liquid behaves like molecular solvents such as acetonitrile and dimethylfomamide. It is found that aromatic chlorides follow a stepwise mechanism, whereas concerted electron transfer/bond cleavage is the preferred reaction mechanism for alkyl and benzyl chlorides. Ag and Cu show catalytic effects only when the DET follows a concerted mechanism, but Ag proves to be a much better electrocatalyst than Cu. A series of substituted benzyl chlorides (Z-C6H4CH2Cl, Z = H, 3-OCH3, 3-F, 4-Cl, and 3-CF3) show interesting results providing some insight into the reaction dynamics. The process occurs by a concerted mechanism and, albeit a constant standard potential for the whole series, Ep on GC and Cu, which does not show catalytic activity, is significantly affected by the substituents. In contrast, Ag shows good catalytic activity and, as expected, Ep does not change with the substituent. This difference in behavior may be rationalized by considering ion–dipole interactions between R˙ and Cl− as opposed to adsorption of the fragments on the electrode surface.