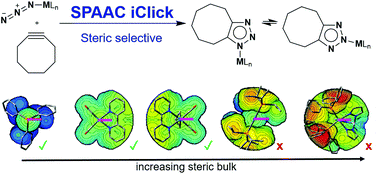
Combining strain-promoted azide–alkyne cycloaddition (SPAAC) and inorganic click (iClick) reactivity provides access to metal 1,2,3-triazolates. Experimental and computational insights demonstrate that iClick reactivity of the tested metal azides (LM-N3, M = Au, W, Re, Ru and Pt) depends on the accessibility of the azide functionality rather than electronic effects imparted by the metal. SPAAC iClick reactivity with cyclooctyne is observed when the azide functionality is sterically unencumbered, e.g. [Au(N3)(PPh3)] (Au–N3), [W(η3-allyl)(N3)(bpy)(CO)2] (W–N3), and [Re(N3)(bpy)(CO)3] [bpy = 2,2′-bipyridine] (Re–N3). Increased steric bulk and/or preequilibria with high activation barriers prevent SPAAC iClick reactivity for the complexes [Ru(N3)(Tp)(PPh3)2] [Tp = tris(pyrazolyl)borate] (Ru–N3), [Pt(N3)(CH3)(PiPr3)2] [iPr = isopropyl] (Pt(II)–N3), and [Pt(N3)(CH3)3]4 ((PtN3)4). Based on these computational insights, the SPAAC iClick reactivity of [Pt(N3)(CH3)3(P(CH3)3)2] (Pt(IV)–N3) was successfully predicted.