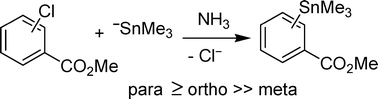
Reactions of methyl 2,5-dichlorobenzoate, methyl 4-chlorobenzoate, methyl 2-chlorobenzoate and methyl 3-chlorobenzoate with Me3Sn− ions gave the corresponding substitution products by an SRN1 mechanism. Competition experiments showed that the relative reactivity of chlorine as the leaving group with respect to the ester group in methyl chlorobenzoate is para ≥ ortho ≫ meta toward Me3Sn− ions. Theoretical studies were able to explain the observed reactivity on the basis of the energetic properties of the transition states of the radical anions formed in these reactions.