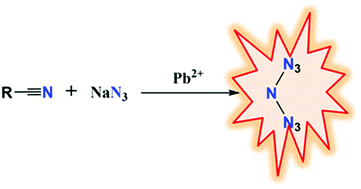
Under hydrothermal conditions, an open-chain N73− anion stabilized in a metal–organic framework (MOF) was achieved for the first time via the in situ reaction of 4-fluorobenzonitrile and sodium azide with Pb2+ ion as catalyst. The anion with C2h symmetry in the MOF was studied by FT-IR, single-crystal XRD and theoretical calculations. Thermal analysis results demonstrated the stability of the anion in the MOF below 430 °C and a high energy content of 8.61 kJ g−1. The anion is also a good reducing agent. It can easily react with basic KMnO4 solution. Moreover, the present study indicates that the Pb2+ ion activates the azide rather than nitrile in the in situ reaction of nitriles with azides to form polynitrogen and this mechanism is a distinct contradiction with the previous results in which the nitrile reacts with azide in the presence of transition metal ions. Our findings may open a new avenue towards the synthesis and capture of polynitrogen compounds.