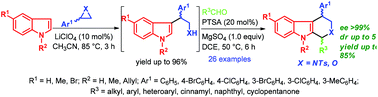
A simple and efficient synthetic route to various 1,4-disubstituted tetrahydro-β-carbolines and tetrahydropyrano[3,4-b]indoles in high yields and stereoselectivity via LiClO4-catalyzed SN2-type ring opening of aziridines and epoxides with indoles followed by p-toluenesulfonic acid (PTSA) catalyzed Pictet–Spengler reaction is described.