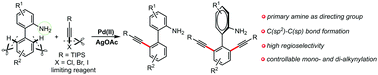
The first example of the palladium-catalyzed primary amine-directed C(sp2)–H alkynylation of biaryl-2-amines has been developed by using (bromoethynyl)triisopropylsilane as an alkynylating reagent. This protocol exhibits a broad substrate scope, excellent regioselectivity and gram-scale synthesis. Significantly, the versatility of this straightforward method was further demonstrated by controlled mono- and di-alkynylation.