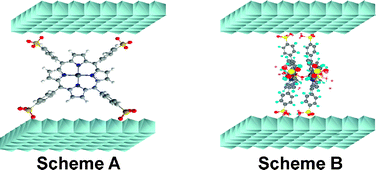
In this study, inorganic–organic hybrids were prepared by intercalation of metal complexes (Zn and Fe) of 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin (TSPP) into Zn2Cr layered double hydroxides (LDH) by coprecipitation method at constant pH. The resulting materials were characterized by powder X-ray diffraction, UV-Vis and FTIR spectroscopies showing the intercalation of porphyrins between the ZnCr layers. While monomer species are intercalated in the case of Zn2Cr-ZnTSPP, the intercalation of dimers is likely to occur in the case of Zn2Cr-FeTSPP. In both materials, a perpendicular orientation of the porphyrin ring against the hydroxide layers is proposed. Of particular interest is the good stability of ZnCr-LDH host over a wide pH range. The electrochemical behavior of these hybrid materials in both neutral (pH 7.0) and acid media (pH 4.5) was investigated by cyclic voltammetry. Zn2Cr-FeTSPP exhibits an electrocatalytic activity for the reduction of oxygen, hydrogen peroxide and nitrite.