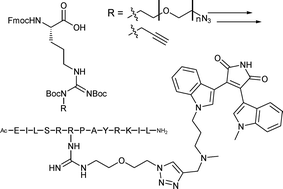
Efficient strategies for the introduction of arginine residues featuring acetylene or azide moieties in their side chains are described. The substituents are introduced in a way that maintains the basicity of the guanidine moiety. The methodology can be used e.g. for non-invasive labeling of arginine-containing peptides. Its applicability is demonstrated by the introduction of ‘click’ handles into a Protein Kinase C (PKC) pseudosubstrate peptide, and the subsequent preparation and evaluation of a novel bisubstrate-based inhibitor based on such a peptide.