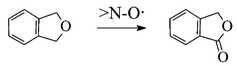
The oxidation of ethers of various structure by O2 with two laccase/![[double bond splayed left]](http://www.rsc.org/images/entities/char_e009.gif) N–OH systems (i.e., HBT, 1-hydroxybenzotriazole, and HPI, N-hydroxyphthalimide) is described. The process affords carbonylic products in reasonable-to-good yields. The oxidation is carried out by the intermediate
N–OH systems (i.e., HBT, 1-hydroxybenzotriazole, and HPI, N-hydroxyphthalimide) is described. The process affords carbonylic products in reasonable-to-good yields. The oxidation is carried out by the intermediate ![[double bond splayed left]](http://www.rsc.org/images/entities/char_e009.gif) N–O˙ species of the mediators, through a radical H-atom abstraction (HAT) route that represents an elaboration of the HAT route followed with benzyl alcohols. Alternative mechanisms have been considered and dismissed. A comparison with a similar oxidation of ethers by the laccase/TEMPO system, reported previously, is made. A clear-cut specialisation of the mediator vs. the substrate emerges, i.e. the laccase/HBT system is more competent for the oxidation of ethers, whereas the laccase/TEMPO system was most proficient in the oxidation of benzyl alcohols.
N–O˙ species of the mediators, through a radical H-atom abstraction (HAT) route that represents an elaboration of the HAT route followed with benzyl alcohols. Alternative mechanisms have been considered and dismissed. A comparison with a similar oxidation of ethers by the laccase/TEMPO system, reported previously, is made. A clear-cut specialisation of the mediator vs. the substrate emerges, i.e. the laccase/HBT system is more competent for the oxidation of ethers, whereas the laccase/TEMPO system was most proficient in the oxidation of benzyl alcohols.
![[double bond splayed left]](http://www.rsc.org/images/entities/char_e009.gif) N–OH systems (i.e., HBT,
N–OH systems (i.e., HBT, ![[double bond splayed left]](http://www.rsc.org/images/entities/char_e009.gif) N–O˙ species of the mediators, through a radical H-atom abstraction (HAT) route that represents an elaboration of the HAT route followed with
N–O˙ species of the mediators, through a radical H-atom abstraction (HAT) route that represents an elaboration of the HAT route followed with