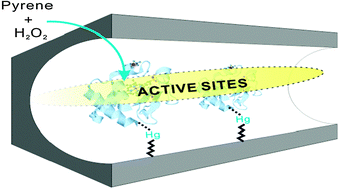
We report a hydrothermally stable and highly reactive cytochrome c (cyt c) immobilized in the nanochannels of mesoporous silica (SBA-15) through a metal affinity interaction. Due to the strong affinity of mercury–sulfuric bonds, we modified the SBA-15 surfaces with 4-aminophenylmercuric acetate (APMA) groups. As a result, an enzyme molecule (yeast cyt c) with a cysteine residue (cys-102) demonstrated strong adsorption, which provided high cyt c loading amounts, highly catalytic activity, and high stability against hydrothermal processes and organic solvents. To compare the immobilization of cysteine-containing cyt c through metal affinity interactions and a traditional covalent bond (a disulfide bond), we modified the SBA-15 surfaces with 3-mercaptopropyl-trimethoxysilane (MPTS) for further production of a disulfide bond with the cysteine residue of cyt c. The cysteine residue of the cyt c can covalently link to thiol-modified SBA-15 through the formation of a disulfide bond. In addition, a non-specific coordination from the thiol groups of SBA-15 to the heme Fe(III) of cyt c may destroy the catalytic center and cause the leaching of Fe(III) ions. Our previous studies of the molecular model have shown that the immobilization of cyt c through the cysteine residue can provide a correct orientation of the catalytic center, where the active site can easily approach the substrate molecules. Therefore, we have developed rapid and highly efficient approaches to immobilize a cysteine-containing enzyme through APMA ligands, which can both protect the protein folding and control the orientation to optimize the stability and catalytic activity.