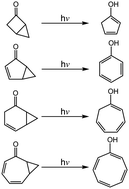
The mechanisms of photochemical rearrangement reactions are studied theoretically using a model system of bicyclic molecules that features a cyclopropane ring that is in proximity to a double bond moiety (such as C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C or C
C or C ![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O group), using the M06-L method and the 6-311G(d,p) basis set. The theoretical findings show that intersystem crossing is vital to successfully interpret the mechanisms. This theoretical research also shows that the photoisomerization mechanism should proceed as follows: Rea-S0 → FC-T1 → Min-T1 → TS1-T1 → T1/S0 → Int1-S0 → TS2-S0 → Int2-S0 → TS3-S0 → Pro-S0. The theoretical evidence in this work confirms the experimental results for both [3.1.0] and [5.1.0] bicyclic molecules. The theoretical observations also predict that for both [2.1.0] and [4.1.0] systems, the quantum yields of the tautomeric keto forms are greater than those of the corresponding enol isomers.
O group), using the M06-L method and the 6-311G(d,p) basis set. The theoretical findings show that intersystem crossing is vital to successfully interpret the mechanisms. This theoretical research also shows that the photoisomerization mechanism should proceed as follows: Rea-S0 → FC-T1 → Min-T1 → TS1-T1 → T1/S0 → Int1-S0 → TS2-S0 → Int2-S0 → TS3-S0 → Pro-S0. The theoretical evidence in this work confirms the experimental results for both [3.1.0] and [5.1.0] bicyclic molecules. The theoretical observations also predict that for both [2.1.0] and [4.1.0] systems, the quantum yields of the tautomeric keto forms are greater than those of the corresponding enol isomers.