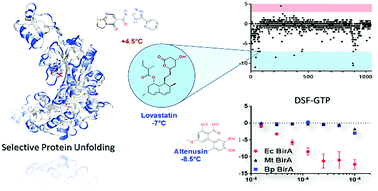
High-throughput differential scanning fluorimetry of GFP-tagged proteins (HT-DSF-GTP) was applied for the identification of novel enzyme inhibitors acting by a mechanism termed: selective protein unfolding (SPU). Four different protein targets were interrogated with the same library to identify target-selective hits. Several hits selectively destabilized bacterial biotin protein ligase. Structure–activity relationship data confirmed a structure-dependent mechanism of protein unfolding. Simvastatin and altenusin were confirmed to irreversibly inactivate biotin protein ligase. The principle of SPU combined with HT-DSF-GTP affords an invaluable and innovative workflow for the identification of new inhibitors with potential applications as antimicrobials and other biocides.