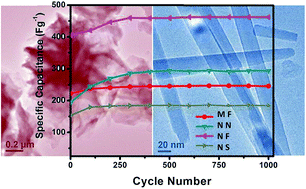
Nickel cobalt oxides (NiCo2O4) superstructures with microsized flowers (MF), nanoflowers (NF), nanoneedles (NN), and nanospheres (NS) have been prepared by a facile self-assembly formation and subsequent conversion process. The various morphologies of the nickel cobalt precursors (NCP) are tuned by using different salts, solvents, surfactants and reaction temperatures. The as-obtained NCP can be readily converted to NiCo2O4 through an annealing process with their structures well retained. The electrochemical properties of the as-derived NiCo2O4 are assessed as the active electrode materials. The results indicate that the NiCo2O4 has exhibited promising pseudo-capacitive properties with high capacitance and good cycling stability. The NF has delivered the highest specific capacitance of 636 F g−1 (at a rate of 1 mV s−1) among all the four samples, showing its potential application for next generation electrochemical capacitors.