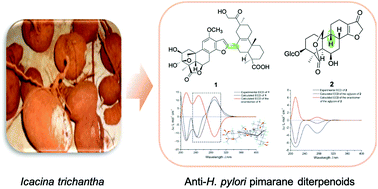
An unprecedented dimeric pimarane-derived diterpenoid (trichanthol A, 1) and a new 17-nor-(9β-H)-pimarane diterpenoid glucoside (trichanthol B, 2) together with ten known analogs (3–12) were obtained from the tuber of Icacina trichantha (Icacinaceae) in a bioassay-guided phytochemical investigation. Trichanthol A (1) is the first example of a pimarane-derived diterpenoid dimer furnished by forming an undescribed C-16–C-7′ linkage, and this is the first report of the glucosylation of a 17-nor-(9β-H)-pimarane diterpenoid (2). Their structures were elucidated by the interpretation of spectroscopic data and chemical methods in combination with calculated 13C NMR-DP4+ analysis and electronic circular dichroism methods. All isolated compounds exhibited antibacterial activity against standard and drug-resistant Helicobacter pylori strains with MIC values ranging from 8 to 64 μg mL−1. Moreover, icacinlactone B (6) showed an additive effect in combination with metronidazole or clarithromycin against H. pylori.