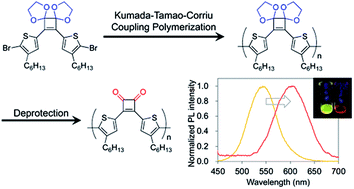
Cyclobutenedione is an aromatic ring that exhibits strong electron-withdrawing properties but is susceptible to undesired reactions with nucleophiles. Herein, Kumada–Tamao–Corriu coupling polymerization of a cyclobutenedione monomer whose carbonyl groups are protected as acetals was achieved. Hydrolysis of the acetals afforded donor–acceptor type π-conjugated polymers consisting of cyclobutenedione as an acceptor unit and bithiophene as a donor unit. The acetal-protected monomer was also subjected to Suzuki–Miyaura coupling polymerization. The absorption and emission spectra of the deprotected polymers shifted to the longer wavelength compared with the acetal-protected polymers.