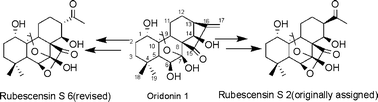
An effective two step transformation of oridonin to 15,16-seco-ent-kaurane skeleton is reported. We also achieved the conversion of one intermediate to natural product rubescensin S and revised its structure as a 13S configuration although 13R is reported in the literature.