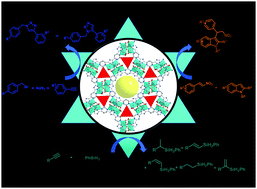
Organic reactions using metal–organic frameworks (MOFs) as catalysts are promising with regard to their environmentally friendly features and potential catalyst recyclability. A robust Co(II)-MOF {[Co2(L-mac)(4,4-bpt)(H2O)]·3.5H2O}n (1) and its enantiomer {[Co2(D-mac)(4,4-bpt)(H2O)]·3.5H2O}n (2) (L/D-mac = basic forms of L/D-malic acid, 4,4-Hbpt = 3,5-di(pyridin-4-yl)-4H-1,2,4-triazole) have been gram-scale prepared under solvothermal conditions. Structural analysis reveals that mac manages Co(II) ions to form 1-D chains, which are further extended via 4,4-bpt connectors into a noninterpenetrating 3D framework architecture. It was found that 1 can be as a heterogeneous catalyst for multiple organic reactions, such as azide-alkyne cycloaddition and Friedel–Crafts reactions with good isolated yields and good recycle runs (at least five times without substantial degradation). Additionally, 1 can promote hydrosilylation of alkynes under harsh conditions with moderate yield.