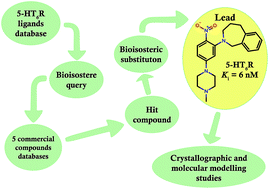
A bioisosteric strategy was successfully implemented with a screening protocol for new, potent 5-HT6R ligands. Initially, 2-[5-(4-methylpiperazin-1-yl)-2-nitrophenyl]-1,2,3,4-tetrahydroisoquinoline (9) was found in commercial databases using a bioisosteric query (screening 5-HT6R Ki = 128 nM). Then, the hit compound was bioisosterically modified (ring alteration) leading to a novel, high affinity (Ki = 6 nM) 5-HT6R ligand (10). Extensive docking studies followed by structural interaction fingerprint analysis supported by single-crystal X-ray structures of the investigated ligands suggest different binding modes with 5-HT6R models for compounds with varying activity. An alternative anchoring point for protonated amine (D7.36) that has not been previously reported was identified.