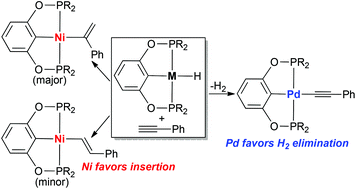
Nickel POCOP-pincer hydride complexes [2,6-(R2PO)2C6H3]NiH (R = iPr, 4a; R = cPe = cyclopentyl, 4b) react with phenylacetylene to generate [2,6-(R2PO)2C6H3]NiC(Ph)![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) CH2 (5a–b) as the major product and (E)-[2,6-(R2PO)2C6H3]NiCH
CH2 (5a–b) as the major product and (E)-[2,6-(R2PO)2C6H3]NiCH![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) CHPh (6a–b) as the minor product. The 2,1-insertion is more favorable than the 1,2-insertion and both pathways involve cis addition of Ni–H across the C
CHPh (6a–b) as the minor product. The 2,1-insertion is more favorable than the 1,2-insertion and both pathways involve cis addition of Ni–H across the C![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) C bond. Unlike the palladium case, alkynyl complexes [2,6-(R2PO)2C6H3]NiC
C bond. Unlike the palladium case, alkynyl complexes [2,6-(R2PO)2C6H3]NiC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CPh (7a–b) and H2 are not produced in the nickel system. The more bulky hydride complex [2,6-(tBu2PO)2C6H3]NiH (4c) shows no reactivity towards phenylacetylene. Catalytic hydrogenation of phenylacetylene with 4a–b takes place at an elevated temperature (70–100 °C) and proves to be heterogeneous. The structures of 5b, 6a, 7a and 7b have been studied by X-ray crystallography.
CPh (7a–b) and H2 are not produced in the nickel system. The more bulky hydride complex [2,6-(tBu2PO)2C6H3]NiH (4c) shows no reactivity towards phenylacetylene. Catalytic hydrogenation of phenylacetylene with 4a–b takes place at an elevated temperature (70–100 °C) and proves to be heterogeneous. The structures of 5b, 6a, 7a and 7b have been studied by X-ray crystallography.