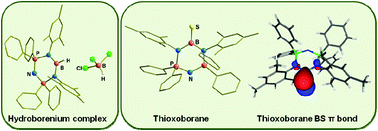
The reaction of a recently synthesized dihydroboron species complexed with bis(phosphinimino)amide, LBH2 (1), (L = [N(Ph2PN(2,4,6-Me3C6H2))2]−) with 3 equivalents of BH2Cl·SMe2 or one equivalent of BCl3 affords the first stable monohydridoborenium ion, [LBH]+[HBCl3]− (2) that is stable without a weakly coordinating bulky anion. Compound 2 can also be prepared directly by refluxing LH with 3 equivalents of BH2Cl·SMe2. Interestingly, reaction of LBH2 (1) with elemental sulfur and selenium involves oxidative addition of S and Se into B–H bonds and subsequent release of H2S (or H2Se) from the intermediate LB(SH)2 (or LB(SeH)2) species forming stable compounds with terminal boron–chalcogen double bonds LB![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) S (3) and LB
S (3) and LB![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) Se (4). The electronic structures of compounds 2, 3 and 4 were elucidated by high resolution mass spectrometry, multi-nuclear NMR and single crystal X-ray diffraction studies. Ab initio calculations on 3 are in excellent agreement with its experimental structure and clearly support the existence of the boron–sulfur double bond.
Se (4). The electronic structures of compounds 2, 3 and 4 were elucidated by high resolution mass spectrometry, multi-nuclear NMR and single crystal X-ray diffraction studies. Ab initio calculations on 3 are in excellent agreement with its experimental structure and clearly support the existence of the boron–sulfur double bond.