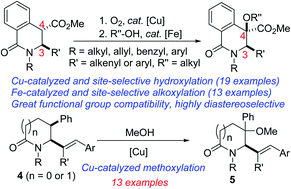
The C–H bond functionalization of sp3 carbon centres presents a significant challenge due to the inert nature of hydrocarbons as well as the need to selectively functionalize one of the numerous aliphatic C–H bonds embodied in organic molecules. Here, we describe catalytic, diastereoselective, and site-selective sp3 C–H hydroxylation/alkoxylation protocols featuring dihydroisoquinolones, γ-, and δ-lactams, which bear vicinal stereocenters. The hydroxylation strategy utilizes oxygen, a waste-free oxidant and affords attractive fragments for potential drug discovery. Fe-catalyzed dehydrative coupling of the resulting tertiary alcohols with simple primary alcohols has led to the construction of highly versatile unsymmetrical dialkyl ethers.