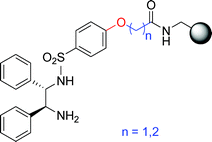
Polymer-supported chiral ligands 9 and 17 were prepared based on Noyori's (1S,2S)- or (1R,2R)-N-(p-tolylsulfonyl)-1,2-diphenylethylenediamine. The combination with [RuCl2(p-cymene)]2 has been shown to exhibit high activities and enantioselectivities for heterogeneous asymmetric transfer hydrogenation of aromatic ketones (19a–c) with formic acid–triethylamine azeotrope as the hydrogen donor, whereby affording the respective optically active alcohols 20a–c, the key precursors of chiral fluoxetine. As exemplified by ligand 17 for substrate 19c, the catalysts can be recovered and reused in three consecutive runs with no significant decline in enantioselectivity. The procedure avoids the plausible contamination of fluoxetine by the toxic transition metal species.