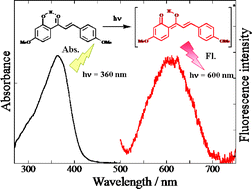
Tautomer fluorescence in the longer wavelength region at 600 nm produced by intramolecular hydrogen atom transfer was observed in several 2′-hydroxychalcones having an electron donating group at the para position of the phenyl ring. The quantum yield of tautomer fluorescence increased by 1000 times upon decreasing the temperature from room temperature to 77 K. The introduction of dendritic substituents also increased the intensity of the tautomer fluorescence. One can control the photochemical and photophysical properties of the olefins by introduction of intramolecular hydrogen bonding and photoinduced hydrogen atom transfer.