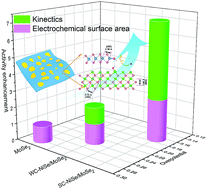
Hydrogen generation through electrochemical water splitting is a promising strategy for renewable energy conversion and storage. Recently, the hydrogen evolution reaction (HER) in alkaline solution has attracted great interest due to its essential role in achieving efficient overall water splitting. In this study, we reported that manipulating the interface configuration could dramatically promote the hydrogen evolution reaction in alkaline solution. Taking two kinds of NiSe/MoSe2 hybrids as examples, we found that strongly coupled NiSe/MoSe2 (SC-NiSe/MoSe2) with strengthened interfacial effects could bring the reduction of overpotential of about 110 mV at 10 mA cm−2 and 7.2 fold enhancement of activity in comparison to separate MoSe2, which has been evidenced by HRTEM, XAFS and DFT results. Furthermore, SC-NiSe/MoSe2 grown on carbon fiber paper shows excellent HER activity with an overpotential of only 71 mV at 10 mA cm−2 and 129 mV at 100 mA cm−2, rendering it a promising electrocatalyst for future application. Our work stresses the importance of making full use of interfacial effects by exquisite construction of microstructures of catalysts, and supplies guidance for the rational design of catalysts.