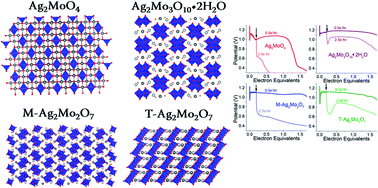
A percolating network of high electrical conductivity needed to operate electrodes at a fast rate can be formed by in situ reduction of Ag+ originating from mixed metal oxide lattices, but few studies have elucidated trends in this mechanism as a function of Ag+ concentration and structure. Candidates compared for the first time here are spinel Ag2MoO4, monoclinic and triclinic Ag2Mo2O7, and Ag2Mo3O10·2H2O, which have reduction potentials for Ag+ and Mo6+ strongly decoupled by up to ∼600 mV in aqueous zinc-ion electrolyte. Under these conditions, Ag0 is the first reduction product and a decrease of charge transfer resistance by ∼100× is observed within 2.5% consumption of total Ag+ independent of initial structure. However, resistance metrics alone poorly describe materials which are robust to reducing silver with high energy at faster rates. Instead, after accounting for crystallinity and morphology differences, we find that the acidity of the molybdate framework is responsible for a switch in charge balance mechanism from the bulk formation of a mixed ZnMoOx to pseudocapacitive Zn2+ precipitation, and that this mechanism switch is associated with minimized losses to rate, voltage and capacity yields as carbon/binder free electrodes relative to composites. The location of this acidity cutoff near the pH of the ZnSO4 electrolyte may suggest a design principle for future low-carbon electrodes beyond molybdate framework structures.